An auto-injector device used used for treating allergic emergencies is being recalled because it might not work when needed, with potentially fatal consequences.
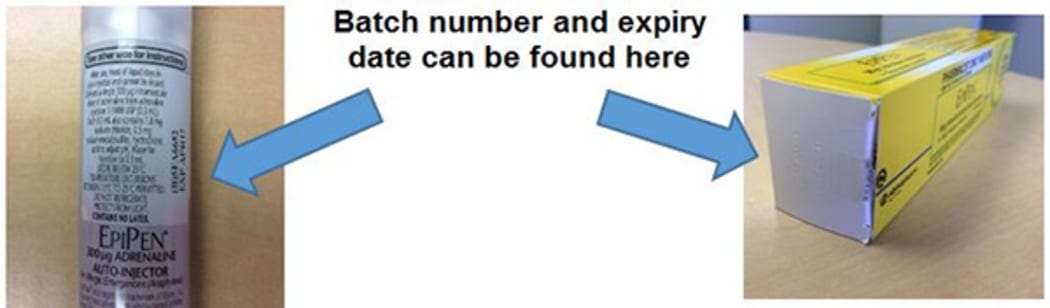
Batch numbers and expiry dates on the recalled products can be found at the end of the carton or on the label of the pen. Photo: Supplied
Mylan New Zealand says that after consultation with Medsafe and the Ministry of Health, it is calling back two specific batches of the EpiPen 300 microgram Adrenaline Auto-Injectors.
It said they could contain a defective part that may result in the device failing to activate or requiring increased force to activate.
Two reports overseas have been confirmed of the device failing to activate from a production run of 80,000 devices, the company said.
From this production run, 1794 had been distributed in New Zealand.
Failure of the auto-injector to activate could mean people did not get the required dose of adrenaline resulting in the worsening of symptoms of anaphylaxis or anaphylactic reactions which could be life threatening.
Peopel need to return the epipens to their pharmacist, who will replace them for free.
The batch numbers are 5FA665 and 5FA6652, both with expiry dates of 17 April.
Consumers can call 0800 335 336 for further information and support.

